Pre-BIO-Europe 24 Primer Sunday November 3
Join us on Sunday prior to BIO-Europe Our annual Pre-BIO-Europe BYO primer will be
We have a range of services specifically tailored to the needs of business development and licensing pros:
We cover and comment on interesting news and trends in our industry
Join us on Sunday prior to BIO-Europe Our annual Pre-BIO-Europe BYO primer will be

How much does it cost to develop a drug? We still don’t know. The
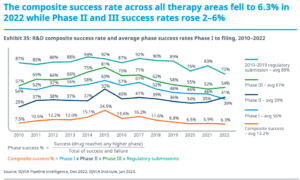
This article was triggered by a beverage-induced discussion I had with Martin Eglitis at BIO-Europe earlier
Collectively, we have decades of experience in consulting and business development. Let us bring this experience to help you solve your challenging in-licensing and out-licensing problems.