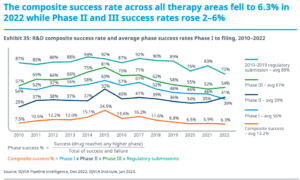
Does Management Matter?
This article was triggered by a beverage-induced discussion I had with Martin Eglitis at BIO-Europe earlier
We have a range of services specifically tailored to the needs of business development and licensing pros:
We cover and comment on interesting news and trends in our industry

This article was triggered by a beverage-induced discussion I had with Martin Eglitis at BIO-Europe earlier
Congratulations! You have successfully advanced your drug candidate to the point where it is

The US FDA recently published an interesting discussion paper, entitled Using Artificial Intelligence &
Our newsletter contains information on current projects, original articles, and information on products or services we use and endorse. It is completely free, and you can unsubscribe anytime.
Collectively, we have decades of experience in consulting and business development. Let us bring this experience to help you solve your challenging in-licensing and out-licensing problems.