An article by our good friend Derek Hennecke in the September issue of Drug Development & Delivery really struck a nerve with us…but in a good way.
To quote (with our emphasis):
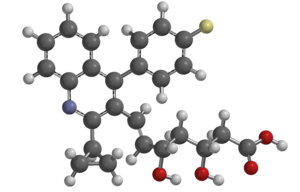
An essay in the Journal of the American Medical Association published in February of last year argued that drugs seeking FDA approval should demonstrate not just non-inferiority, but superiority compared to available drugs. The article lambasted the FDA for approving pitavastatin (Livalo), resulting in eight statins approved for use in the US. The author contends that eight is too many, and that new drugs should be at least safer, more efficient, or suitable for other populations, such as the elderly or children.
Such a move would hamper innovation further. No drug company ever sets out to develop a me-too drug. They always start with a molecule they believe is better. When it turns out the drug’s efficacy is similar to an existing drug, does that really mean this new molecule should be thrown out with the bathwater? There are so many reasons to let these drugs come to market. Doctors, for one, are always on the lookout for precisely the kind of options these drugs present. When a patient doesn’t respond to one drug, or perhaps reacts with a nasty side effect, doctors need options to be able to match the patient to the drug that performs best for him or her. Perhaps even more importantly, shutting down promising new molecules closes an entire avenue of potential innovation. The availability of any one new molecule could lead to untold transformational discoveries that would never otherwise see the light of day.
was fifth to market?
