Year in Review Video – 2024 Edition
Our Annual Year in Review Video – 2024 Edition At long last, here is
Our Annual Year in Review Video – 2024 Edition At long last, here is
Join us on Sunday prior to BIO-Europe Our annual Pre-BIO-Europe BYO primer will be

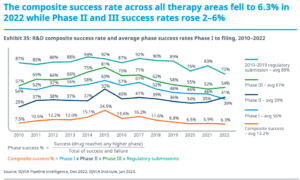
How much does it cost to develop a drug? We still don’t know. The
This article was triggered by a beverage-induced discussion I had with Martin Eglitis at BIO-Europe earlier
Congratulations! You have successfully advanced your drug candidate to the point where it is

The US FDA recently published an interesting discussion paper, entitled Using Artificial Intelligence &

It seems as if nobody talks about anything except AI these days, especially in
Biotech industry poised for boom: 2023 licensing & M&A predictions.
Why don’t companies write business plans anymore? Are they useless? Is it simply faster to put together 10 slides? In this post, we discuss this interesting question.
Here is our annual year in review, along with some predictions (which are rarely

We recently completed a Scouting project on behalf of a geographically-focused company. In this

Like many of you, I am also knee-deep in last minute preparations for BIO.

Consider the following scenario… You are the CEO of an emerging biotech company. You

Passport in date? Check Suits still fit? Too big, but wearable. Negative COVID tests?

…the enduring competitive advantages in a global economy lie increasingly in local things –

Of course we are being facetious when we use the word “scam.” But it

Is this 2020? Consider the following scenario… It is the beginning of the calendar

Many thanks to every one who participated in our recent survey on virtual /

What will our industry and economy look like in the post-COVID-19 era? First of
As our current situation continues to evolve, it is critical that your out-licensing and/or

Empty spaces. What are we living for? Abandoned places. I guess we know the

Will the current coronavirus situation affect biotech and pharma business development? Yes…and No.
Are virtual or digital partnering conferences the wave of the future? Or will we

It is an interesting question. With so much information and mis-information coming at us,

Ophthalmology is changing. If we look back 5-10 years, topical formulations of novel or

Happy New Year, everybody. And with the new year comes the annual report from
Which stories were the most interesting in 2019? And, what should we look forward

Biopharma Dive recently published a series of articles on biotech bankruptcies. The first lists
Like you, we go to a lot of partnering conferences…BIO…BIO-Europe…BioTrinity…and so on. But these
A few months ago, the Centers for Disease Control published a new analysis, concluding

Remember me? Back in the mid-80s, when the Sony Walkman was a cassette player,

Internet pharmacies specializing in erectile dysfunction, hair loss, and contraception are booming. But are they a good idea?

Our recent post entitled “What is confidential?” triggered a few questions and comments via email. Our latest post tackles these questions.

What is confidential? And what should you disclose before and after a CDA is signed?

Want to meet more prospective clients and investors at #BIO2020? Lacerta BIO is offering you the opportunity to sponsor your own event in San Diego.

Is Japan being ignored as a licensing partner in the biotech industry? Is China now getting all of the attention? Lacerta Bio explores this question.
Undoubtably many of you have seen this graphic as it has made the rounds.
What is Digital Scouting? The term “digital scouting” refers to an increasingly popular
Derek Lowe is a medicinal chemist with ~30-years of industry experience, mainly in Big
Last year was unquestionably an excellent year from an FDA NCE approval perspective. A
That didn’t take long. In mid-December, we released a short video that summarized a
Somewhere in the deep recesses of our collective memory, the Concentration Ratio resides. It’s
BIO recently published their latest report characterizing innovation in highly prevalent, chronic diseases. A
Back during our early days as an Analyst with a venture capital fund, one
Want to spend an entire decade surfing the web? Try finding and reading every
Congrats on beginning your quest towards the Holiest of Grails in our industry…capital. Your
The August Edition of Life Science Leader has a summary of a panel discussion.
The good folks at Pharmaprojects just published their Pharma R&D Annual Review. A
Two months ago, it was reported that six US states filed lawsuits against Connecticut-based Purdue
Conferences like #BIO2018 are challenging, but highly rewarding experiences. This year was especially good,
We are in the early days of a short, but intense consulting project
The coverage of the announced negotiation by Sanofi to divest its European generics business
Last week, an FDA Advisory committee unanimously recommended the approval of Epidiolex for
Last week was the 20th anniversary of the launch of Viagra (sildenafil, Pfizer).
Last week was another jam-packed week of meetings, this time at BIO-Europe Spring
PWC recently published their 12th annual Top Health Industry Issues report for 2018. The
Following up on their sobering report on drug development in depression, BIO has
If you have nothing better to do on a cold Winter’s day, try researching
Last week, BIO published an exceedingly interesting report, entitled The State of Innovation in
Dr. Eric Topol recently posted an interesting image on Twitter. According the US
Deloitte recently published their annual report on returns generated by big pharma from
As a pharmaceutical scientist, the concept of “synthetic biology” sounds somewhat oxymoronic. It’s
What did we find interesting about 2017? What will happen in 2018? Our
As of this writing, there are 100 days until we’re wheels up and
It’s amazing how months of preparation for a conference are realized in 4-5
Simple question…is our industry risk averse? Some would say, “Yes, absolutely.” Look at psychiatric
The recent news that Novartis is closing its Colorado-based generics manufacturing facility brought back
Is out-licensing difficult? Yes, of course it is. But how difficult is it? Is
This post is in response to a prospective client, who asked us about the
The humble lime…sour, sweet, juicy, and an essential ingredient in many desserts and beverages.
Over on LinkedIn, we posted an article on long form lectures/presentations. In that article,
Countless electrons have been spilled in the promotion of the biotech and life science
According to Wikipedia, Management by Wandering Around (MBWA) is …refers to a style of
#BIO2017 is days away. What can we expect? Crowded Partnering – By
An Acceptance Rate is simply the percentage of meeting requests which are accepted
NSAIDs are an $11-12+ billion market, yet the problems with these drugs continue
The following commentary is from Mr. Tom Brya, Managing Director at Titenare GXL.
A recent post by Robert Wessman of Alvogen pointed out that some
We just returned from a brief trip to England, where we met with a
Last week, the US Centers for Disease Control published a rather alarming report
We recently learned about Bayer’s Grants4Apps program. This program provides support to companies and
This is a good question…a question which lacks a simple answer without quite
Yeah, it rained last week in San Francisco. But the rain did not stop
I believe it is fair to say that 2016 was a bit unusual.
The recent elections in the United States is bringing many long-standing policies into question.
Few of us will soon forget waking up in Cologne to the news that
BIO Europe was, yet again, an excellent conference for Lacerta Bio. Having the US
Can we ESKAPE the problem which may kill thousands of human beings over the next
A few weeks ago, we attended the first annual Commercializing Biotech Innovation Conference in Syracuse, New
Last month, the FDA announced their approval of Amjevita, a biosimilar to AbbVie’s Humira. Humira
Our recent trip to Stockholm and the Nordic Life Science Days conference was quite enlightening, especially
In our last post, we presented a model which helps us aggregate a series
A triumvirate is a three-person (or three-group) organization which is established for a
Ample numbers of electrons will be spent discussing the BIO2016 conference in San
Next week, our industry makes an unusual return trip to Union Square, as we
There is an interesting article in the current issue of The Journal of Precision
Our friends at PharmaCircle recently published an interesting and provocative piece entitled Injectables: The
How was Bio Europe in Stockholm last week? The Conference Itself – For us,
We’re late to the this party, but HBM Partners issued their two annual
At last month’s Biotech Showcase, a panel discussion was held discussing different models
Nearly 30 years ago, back when your author was a humble pharmacy intern, the
This morning, our friends at EBD and MacDougall Biomedical Communications presented a very good
Last week, a study published in JAMA Dermatology examined the skyrocketing prices for dermatology
I love what you did with the place… It’s always difficult for us to
We go to a lot of conferences. And, as a result, we see a
Last month, EBD and IMS partnered to analyze meeting outcomes from four conferences, covering
Last week, we had the good fortune of spending a week in India,
As part of my preparations for an upcoming trip to India, my physician prescribed
Michael Gilman, CEO of Padlock Therapeutics, wrote a terrific post recounting his experiences
The July/August issue of Drug Development and Delivery has an extensive report on
This question came up recently when I was describing one of our current
OK, so the new One-on-One Partnering system has not had a stellar introduction.
What follows is a true story. In 2013, we were engaged to out-license a
We just finished reading a terrific academic piece by Jack Scannell and colleagues
Earlier this month, our friends at Biotech and Money published a transcript from
We’ve started preparing for the BIO convention in Philadelphia in June. Preparing? Already?
Are you an independent consultant? Are you looking for a new platform or
Last week Reuters published an article summarizing the “virtual” biotech business model. It
Today, Derek Lowe points out that Shenzhen Chipscreen received CFDA approval for
I was privileged to spend 10+ days traveling to Copenhagen (Denmark), Lund (Sweden),
From a consulting perspective, here is how we view business development, using the acronym
There is a terrific article in Nature Biotechnology entitled Keys to the Kingdom. The
Today’s issue of Institutional Investor has an interesting article on the reality of
Earlier this week, BIO released what we think is a valuable and important
Lisa Jarvis has prepared a handy overview of the new drug approvals in 2014:
Today, Bruce Booth published yet another terrific post, where he discusses the current biotech
It’s hard to believe that a week has passed since we were dashing
The decks have been prepared, edited, and uploaded to the cloud. The meeting schedule
In Part One of his series, Dr. Garrett Lindemann discussed some of the challenges facing Wyoming as it
Mind The Gap… We’re late to this party, but a really good interview of David
The new DiMasi estimates for R&D costs were published this week. You can get a
Earlier this week, The Economist travels over well-trod ground in an article on pharmaceutical
Can’t get there from here… We’re back from another excellent BIO Europe convention.
There’s more to Wyoming than this. In Part One of his series, Dr. Garrett Lindemann
Is this the next major biotech cluster in the US? Wyoming? Yes, Wyoming. Why
The reports of my death have been greatly exaggerated. Mark Twain In July,
Twenty Five. That’s it. What are we talking about? Some of the data in yesterday’s
At Lacerta Bio, we’re big believers in the therapeutic benefits of drug delivery in
This morning, California-based Avanir Pharmaceuticals announced that their combination of dextromethorphan HBr and
Thompson Reuters published a good overview of the challenges facing companies developing biosimilars.
A few weeks ago, BIO conducted an interesting panel discussion as part of
Lacerta Bio is pleased to return to Europe for the 20th Anniversary of the
We’re actively seeking guest bloggers for LacertaBio.com! The ideal guest blogger: – Is
This morning we learned that Sanofi is the winner in the “Where
Yes, and it might be worth it. Yesterday, Daiichi Sankyo announced an incredible $650
Source: Nature Biotechnology, 32, 617–619, (2014), Published online 08 July 2014 Our good
Do you know which are the top branded drug products in India? We
We’re actively seeking guest bloggers for LacertaBio.com! The ideal guest blogger: Is currently
A quick search resulted in several recent examples of US-based pharma companies executing “inversions”
We’re back and knee-deep in BIO2014 follow up work. Lacerta Bio was
An excellent analysis of the AstraZeneca pipeline performance was recently published in Nature
This morning, Thompson Reuters and BIO presented a good webinar on preparing for BIO.
Our friends at Pharmaphorum posted an overview of the recent WHO guidance
Much ink has already been spilled discussing the acquisition of Ranbaxy from
Yesterday, MannKind Corporation annouced that the FDA voted 13-1 in favor of an
This week, the financial press is going bonkers over the acquisition of WhatsApp by
Are we the only ones shocked by this? Despite efforts by the Obama
We’re still knee-deep in our follow up activities from the San Francisco
Yesterday the Wall Street Journal summarized the current situation in antibiotic drug
This week we’re greeted with the following headlines: The FDA Approvals of
Those of us of a certain vintage will remember the days when
We’ve returned from another successful BIO Europe conference, this time in Vienna.
Lacerta Bio is pleased to announce our participation in the
Ed Silverman at Pharmalot reports today that BI is closing Ben Venue
We recently presented a 30-minute live webinar on ways to improve your business development
We’ve posted about the Indian pharmaceutical market before, but this report is
Today we learn that a biogeneric form of Remicade, the first biogeneric
Today we learn that Endo is acquiring Boca Pharmacal for $225 million:
At Lacerta Bio, we believe that business development is a process…a process
How much does it cost to develop an approved drug? We may never know, but Matt Herper from Forbes takes an interesting approach to come up with some estimates.
Texas-based Pernix Therapeutics announced a $30 million divestment of
We don’t normally comment on stock activity, but the market response to
According to Burrill & Company, July had the busiest week of biotech
In a surprise decision last week, the US FDA decided to approve
Many people think of the BRIC countries as good domestic opportunities. But
Ok, we have to admit. This is not exactly a “how to”
Just sign a piece a paper and drive off with a new
Biotech business development has become more sophisticated and more complex. Yet we
It’s been difficult to keep up with all the deals this month!
Oramed announced last week that their oral insulin capsule is ready for
In 1941, Let Us Now Praise Famous Men was published. This unusual
As we were making our way back home from Chicago (on delayed
Yesterday, North Carolina-based Pozen gave a detailed Investor Update, focused on PA32540,
Today’s issue of FiercePharma covered two interesting FDA decisions (or non- decisions, if
Rob Wright has a really good article on preparing for BIO. In
Very interesting point made by Ranjit Shahani from Novartis India: The immense talents
At Lacerta Bio, we’ve been reading the fascinating book, The Signal and The Noise
The always excellent Bruce Booth wrote a good summary of the key Preclinical deals we’ve seen
As BD consultants, we attend a number of conferences, both large and small. Some
Today we learn that generics giant Mylan has entered into a partnership with India-based
The announcement of the Allergan acquisition of MAP Pharmaceuticals for nearly $1 billion (and the NuPathe approval)
Lacerta Bio, along with approximately 10,000 other life science investors, executives, consultants, service providers,
Happy New Year! For many companies, 2012 was a challenging year. How will 2013
Consider the following situation: You are out-licensing an innovative asset in a niche
Last week, Sandoz announced they are closing a number of generic product development centers
While we were recovering from our turkey-induced slumbers, we read the shocking news that
We’ve had the good fortune to work with a biosimilars client this year. Working
As we’ve done in the past, we’ve tabulated the countries of origin from the
We’ve returned from BIO Europe 2012 in Hamburg with a stack of business cards,
Bruce Booth (yet again) published a terrific post on his blog, entitled “NextWave of
There are some very interesting data published yesterday on FDA approval statistics: It was
We spent a few days in Chicago attending the AAPS Convention. As usual, it
Next week, Lacerta Bio will be at the AAPS Annual Meeting in Chicago. If
When we studied pharmacy, compounding was in integral part of our education. On a
An intriguing graph was posted on Twitter the other day: Now we understand the
An article by our good friend Derek Hennecke in the September issue of Drug
Pharmaceutical and biotech competitive intelligence is increasingly important and challenging these days. Lacerta Bio discusses a recent case study in which a client learned this the hard way.
Lacerta Bio is pleased to announce our attendance at the upcoming BIO-Europe 2012
In response to the recent acquisition of Medicis by Valeant, an interesting poll underway
At Lacerta Bio, we’re in the middle of several international out-licensing projects. This process
We generally agree with the notion that many early-stage biotechs should be structured as
As we read the continuing bad news regarding venture financing in biotech, we came
On Friday, it was announced that the former Pfizer R&D center in Kent, UK,
We were disappointed to read this yesterday: Pfizer ($PFE) and Johnson & Johnson ($JNJ) have
Thank goodness for Twitter. How else can one keep up with all of the
A recent article in Forbes by ex-Pfizer President or R&D John LaMattina asks a
Our good friend Raman Minhas at ATPBio has a really good post today on
As with BIO 2011, the bulk of our time was spent in the One-on-One
While we were all burning shoe leather at BIO2012 this week, the good folks
One again, Bruce Booth published a terrific post entitled Contrarian Opportunities in Biotech Venture.
In researching our latest project, we came across some startling statistics. A report published
So said Shams Ruston from Labopharm at last week’s Interphex conference in New York.
Yesterday, we responded on LinkedIn to the following note: THis is our first BIO
AOL sells patents to Microsoft. Why? The reasons for AOL selling these patents are
The past seven days have brought us two very interesting transactions. First, on March
Earlier this week we learned that Pfizer will return to Biocon rights to biosimilar
The February, 2012 issue of Life Science Leader has a wide-ranging interview with G.
UPDATE (21 February) – Here is a decent video interview of Rachel Sherman, head of
Last week, the NY Times published an interesting article on the cause of Alzheimer’s
Lacerta Bio has returned to cold, windy New York following a solid week in
Last month, Lacerta Bio celebrated its first full year in existence. In fact, our
Happy New Year, everyone! Next week, 2012 kicks off with the madness otherwise knows
We are busy preparing for the Annual Industry kickoff conference at the Westin in
Lacerta Bio was privileged to give a short talk at the first (and hopefully
Pharmaceutical outsourcing to CROs is certainly nothing new. However, this week we learned that
Lacerta Bio was pleased to take part in a terrific overview of technologies for
This week, our good “acquaintances” from EBD Group launched partnering360. What is it? According
Lacerta Bio spent the week at the AAPS ( American Association of Pharmaceutical Scientists
Call us late to the party, but in August of this year, KPMG published
In our view, there are two basic types of biotech business development-related due diligence.
Earlier this month, Keith Powell posed an interesting question: The economies of California and
What do companies such as Twitter, Facebook, and Google have in common with Pfizer,
Some members of the media are ebullient with the news that Merck is “getting
Lacerta Bio was fortunate to attend this year’s BioPharm America conference in Boston. Here
Yesterday’s Financial Times reported that pharmacists in Spain are struggling to continue operating their
GSK announced that it has taken a ~25% stake in a new spin out.
On the one hand, you hate to see talent leaving the US. But, you
Posts have been light lately, due to vacations, summer hours, etc. However, we’ve remained
Today’s New England Journal of Medicine contains a short article by Kozlowski et al
As we were wrapping up our annual July vacation, we were shocked to hear about the
As FiercePharma correctly points out, GSK’s Q II report reflects the overall industry trends
Today, KPMG is reporting results from a survey of pharmaceutical executives. In this survey,
Luke Timmerman at Xconomy wrote a provocative piece today entitled The Missing Ingredient in Today’s Biotech: Guts.
We previously posted our comments on BIO 2011. However, one aspect of the conference
The Lacerta Bio team attended the annual BIO Convention in Washington, DC last week.
The pharmaceutical industry has benefitted mankind by developing life-saving and life-sustaining drugs. However, recent turmoils have led many to question the value provided by the industry. Matt Herper tackles these complex issues across two blog posts.
The June, 2011 issue of Nature Drug Discovery has a interesting short article based
We posted in February the news that Pfizer was closing their large R&D facility
Bruce Booth from Atlas Ventures has a fascinating story on his blog. We won’t
There is a fascinating discussion taking place over at Derek Lowe’s blog today on
Today we learn that Rigel has raised $130 million in financing, in part, to
Flowtown has a terrific (and large!) graphic entitled “The New Marketing Trifecta.” The trifecta refers to
Bloomberg has a good round up of several emerging pharmaceutical companies developing antibiotics. As
There is an excellent editorial in the May issue of Nature Biotechnology entitled Inadequately
Today’s In Vivo Blog post summarizes comments that we have been hearing from our
This is a fantastic piece in Nature by Anu Acharya, CEO of Ocimum Biosolutions
This week we learn that two novel pain medications will never see the light
We regret that we were unable to attend the panel discussions at BioTrinity last
Last week, Lacerta Bio attended the annual BioTrinity Partnering and Investment Conference in Newbury,
Fred Frank, a highly respected investment banker, greeted us on Tuesday morning with this missive:
It was recently announced that the EMA has rejected Xyrem for the treatment of
This weekend was spent attending the Natural Products ExpoWest convention in Anaheim on behalf
Drug development costs of $1 billion are often quoted. A new study estimates costs to be much lower, based on a different statistical approach.
We were intrigued by a comment made on Twitter regarding pharma employee involvement with
This evening, @AstraZenecaUS held a fascinating Twitter chat (hashtag #RxSave). The focus of the
There has been a lot of commentary this week on Pfizer’s announcement that their
There is a lot of good commentary and analysis of last week’s JP Morgan
In our meetings and informal discussions, there were three items we heard again and
The past few months have seen a number of reports describing how national governments
Earlier this week we wrote about the emerging trend of increasing state government involvement
Like many of you, we are already scheduling meetings at the annual JP Morgan
Our good friends at Drug Delivery Technology have, as of January, changed the name
Last week we wrote about the Russian government’s desire to grow their national pharmaceutical
Here is a sample of announcements from today alone regarding the pharmaceutical industry in
Earlier this week, Prime Minister Putin announced that the Russian government will invest nearly
There is fascinating news today from Pfizer, who have decided to bypass the wholesalers in
The EMA has recently published guidelines for the development of monoclonal antibody-containing biosimilar products.
BioPharm Int’l recently published a nice overview of where the FDA debate is on
The Indian pharmaceutical market is highly fragmented. How can pharma companies make money? PharmaTimes characterizes the market for us in summary form.
PharmaPhorum has an excellent analysis on launch evolution across pharmerging markets, such as China,
Today we learn that Lilly is acquiring Avid Radiopharmaceuticals: Lilly will acquire all outstanding shares
Fascinating turn of events on Friday, when the DOJ: said on Friday that human
The October, 2010 issue of Life Science Leader has an interesting article entitled Orphan
A former business development colleague of mine used to refer to some prospects as
Today we learned that Lilly will close their Singapore R&D facility. This facility was
It is regrettable that the FDA issued a complete response letter to Jazz Pharmaceuticals
Physicians typically don’t interact with their patients via email. This is understandable and quite interesting, as physicians tend to be quite computer savvy. A recent report explains the reasons why this is the case.
Drug delivery is not dead! In fact, drug delivery can ba a valuable tool for developing and commercializing drugs faster and at lower cost versus NCEs.
Late last month, Johnson and Johnson (J&J) announced the results of Phase III clinical
Today we read the news that GSK will spin out a team of fourteen
Chronic pain patients have to wait even longer for access to twice-daily tapentadol.
It’s certainly interesting to consider Latin American countries from a US perspective. Aside from
Today we hear the news that Covance has done it again. Today they acquired